How Do Hydrogen Cars Work? Your Friendly Beginner’s Guide to the Future of Driving?
Forget gas stations and lengthy charging cables. Imagine filling your car in minutes and emitting only pure water vapor. That’s the promise of hydrogen fuel cell vehicles (FCFVs or HFCVs). But how do these futuristic machines actually work? Let’s break it down, step-by-step.
The Big Idea: Electricity from Hydrogen & Oxygen
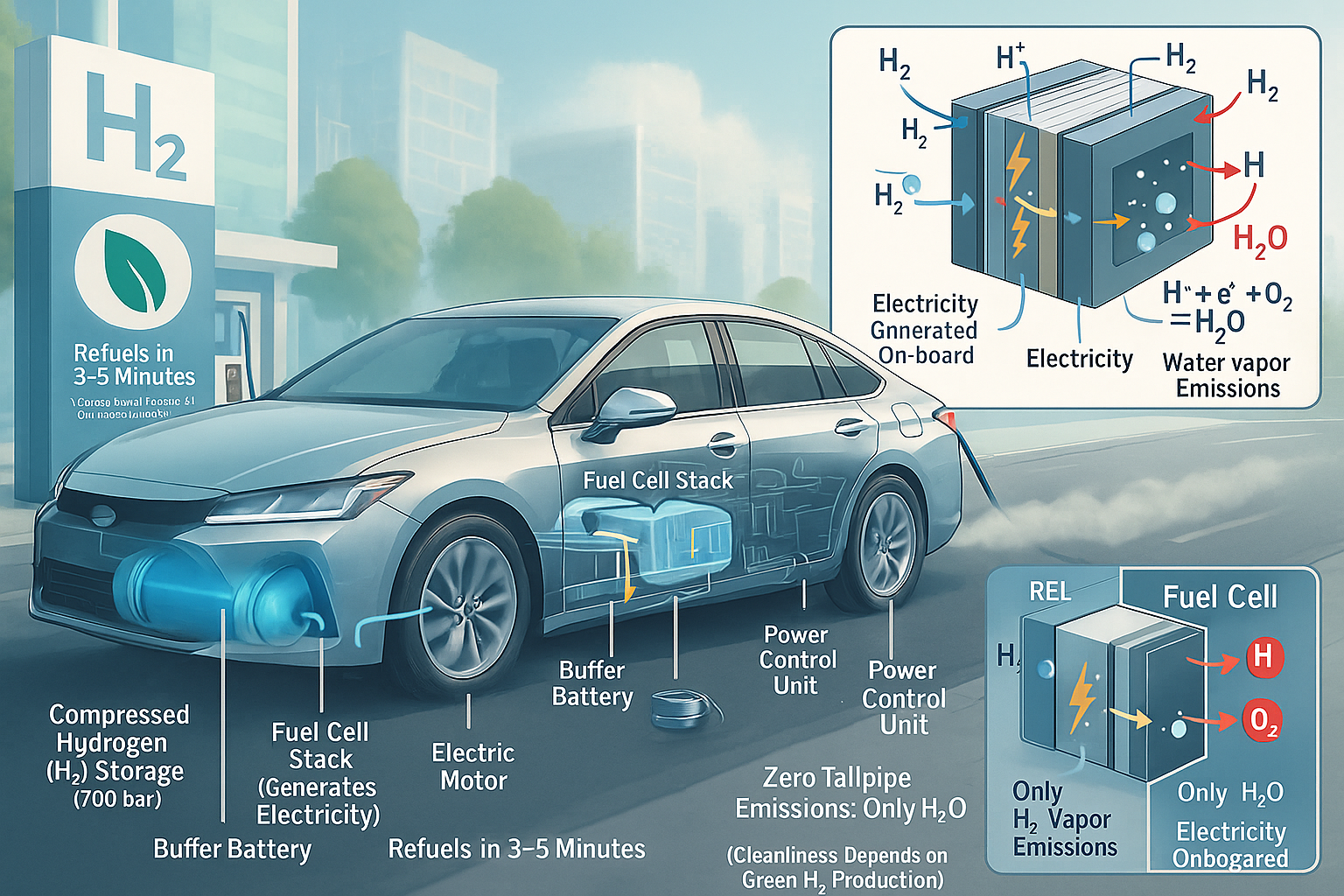
At its heart, a hydrogen car is an electric vehicle (EV). But instead of storing electricity in a large battery pack charged from the grid, it generates its own electricity onboard using hydrogen gas and oxygen from the air. This happens through an electrochemical reaction inside a device called a Fuel Cell Stack.
Key Components Under the Hood (or Floor):
- Hydrogen Fuel Tanks:
- What: High-pressure, ultra-strong tanks (usually carbon-fiber reinforced) storing compressed hydrogen gas (H₂).
- Why: Hydrogen is very light but not very dense. To store enough for a decent driving range (300-400+ miles is common), it’s compressed to incredibly high pressures – typically 700 bar (10,000 psi!).
- Safety: These tanks are rigorously tested and designed to withstand extreme impacts, fire, and even gunfire. They’re arguably safer than gasoline tanks in many scenarios.
- The Fuel Cell Stack: The Power Generator
- What: The core technology! It’s a stack of many individual fuel cells working together. Each cell contains an Anode (negative side), a Cathode (positive side), and an Electrolyte Membrane in between (commonly a Proton Exchange Membrane or PEM).
- How it Works (The Magic Reaction):
- Hydrogen Delivery: Hydrogen gas (H₂) from the tanks is fed to the Anode.
- Splitting Hydrogen: At the anode, a catalyst (usually platinum) helps split each hydrogen molecule (H₂) into two protons (H⁺) and two electrons (e⁻).
- Proton Journey: The Electrolyte Membrane only allows the positively charged protons (H⁺) to pass through to the Cathode.
- Electron Flow: The negatively charged electrons (e⁻) CANNOT pass through the membrane. Instead, they are forced to travel through an external electrical circuit. This flow of electrons is the electricity that powers the car’s motor!
- Oxygen’s Role: Meanwhile, oxygen (O₂) from the air is fed to the Cathode.
- The Reunion: At the cathode, the protons (H⁺) that travelled through the membrane, the electrons (e⁻) that travelled through the circuit, and the oxygen (O₂) combine. With the help of another catalyst, they react to form water (H₂O) and release heat.
- The Net Reaction: Hydrogen + Oxygen → Electricity + Water Vapor + Heat
- Key Point: No combustion occurs! It’s a clean electrochemical process.
- Electric Motor:
- What: Exactly like the motor in a battery electric vehicle (BEV).
- How: It uses the electricity generated by the fuel cell stack (and sometimes supplemented by a small battery – see below) to turn the wheels. It’s quiet, powerful, and provides instant torque for smooth acceleration.
- Lithium-ion Battery (Small):
- What: A much smaller battery than in a pure BEV.
- Why:
- Buffering & Smoothing: Stores excess electricity from the fuel cell during low-power demands (like cruising) and provides extra power during high demands (like hard acceleration or climbing hills). This helps the fuel cell operate more efficiently.
- Regenerative Braking: Captures energy normally lost during braking, converting it back into electricity stored in this small battery.
- Startup & Ancillary Power: Powers initial systems and accessories when the fuel cell isn’t fully active.
- Power Control Unit (PCU):
- What: The car’s “brain” for electrical power.
- How: Manages the flow of electricity between the fuel cell stack, the battery, and the electric motor. It ensures the right amount of power is delivered when and where it’s needed for optimal performance and efficiency.
- Exhaust:
- What: Simply a pipe.
- What Comes Out? Only water vapor (H₂O) and warm air. That’s it! No tailpipe emissions of carbon dioxide (CO₂), nitrogen oxides (NOx), or particulate matter.
The Driving Experience:
- Refueling: Similar to gasoline. You pull up to a hydrogen station, attach a nozzle, and fill the high-pressure tanks. This takes 3-5 minutes – comparable to filling a gas tank and much faster than charging a BEV.
- Starting Up: The system initializes, checks pressures, and begins feeding hydrogen and air to the fuel cell stack.
- Driving: Pressing the accelerator signals the PCU. The fuel cell stack ramps up hydrogen/air flow to generate the electricity needed. Electricity flows to the motor, turning the wheels. Excess power charges the small battery. The PCU seamlessly blends power from the fuel cell and the battery as needed.
- Braking: Regenerative braking captures energy, storing it in the small battery.
- Stopping: The fuel cell stack can idle down or shut off, with the battery powering accessories.
The Environmental Angle (It’s Nuanced):
- Tailpipe Emissions: Zero. Only water vapor comes out. This is a massive advantage for local air quality.
- “Well-to-Wheel” Emissions: This is crucial. Hydrogen itself isn’t a primary energy source like oil; it’s an energy carrier. How clean an FCEV is overall depends entirely on how the hydrogen fuel was produced:
- Green Hydrogen: Made by splitting water (H₂O) using electricity from renewable sources (solar, wind, hydro). This is the truly clean, sustainable goal. Emissions: Near Zero.
- Grey Hydrogen: Made from natural gas (methane – CH₄) in a process called steam reforming. This is the most common method today. Emissions: Significant CO₂ released during production.
- Blue Hydrogen: Made from natural gas like grey hydrogen, but the CO₂ is captured and stored (Carbon Capture and Storage – CCS). Emissions: Much lower than grey, but not zero (some leakage occurs).
- The Verdict: Hydrogen cars are only as clean as the hydrogen production process. Widespread adoption requires a massive shift to green hydrogen production to realize their full environmental potential.
Pros and Cons at a Glance:
| Feature | Pros | Cons |
|---|---|---|
| Refueling | Fast (3-5 minutes), like gasoline | Very limited hydrogen refueling stations |
| Range | Long (300-400+ miles), comparable to gas | |
| Emissions | Zero tailpipe emissions (only water) | Production emissions depend on H₂ source (Grey = High) |
| Driving | Quiet, smooth, instant torque (like EVs) | |
| Efficiency | Overall “well-to-wheel” efficiency lower than BEVs | |
| Cost | High vehicle cost; Hydrogen fuel cost currently high | |
| Infrastructure | Extremely limited refueling network globally | |
| Production | Scalable green H₂ production is key | Green hydrogen production still nascent/expensive |
The Hydrogen Car Landscape (Examples):
- Toyota Mirai: One of the pioneers and most well-known FCEVs.
- Hyundai Nexo: A popular SUV-style hydrogen vehicle.
- Honda Clarity Fuel Cell: Another early contender (though production has paused).
Are Hydrogen Cars the Future?
Hydrogen fuel cell technology offers compelling advantages: long range, ultra-fast refueling, and zero harmful tailpipe emissions. It’s particularly attractive for larger vehicles (trucks, buses) and applications where long range and quick refueling are critical.
However, significant challenges remain: building out a vast and expensive hydrogen refueling infrastructure, drastically reducing the cost of green hydrogen production, and lowering the manufacturing cost of the vehicles and fuel cells themselves.
The Bottom Line:
Hydrogen cars work by generating electricity onboard through a clean chemical reaction between hydrogen and oxygen in a fuel cell stack. This electricity powers an electric motor, emitting only water vapor. They offer a promising, potentially zero-emission alternative, especially for drivers needing long range and quick refuels. While facing infrastructure and cost hurdles, they represent a fascinating and important part of the diverse toolkit needed to decarbonize transportation. The journey to a hydrogen highway is just beginning!